Flash chromatography is a separation technique used to separate and purify chemicals. Hence, it's also called flash purification because it involves the purification of chemical mixtures. You can use it to purify and separate natural products or novel molecules. This process helps to remove unwanted by-products to ensure you create the desired product, especially in synthetic reactions. Chromatography applies the principle that compounds in a solution separate due to the differences in their polarity. But this only happens under the right conditions and technique. Here's more on how ELSD works and when to use it in flash chromatography.
ELSD and Chromatography
Usually, chromatography requires UV light to detect different compounds in a mixture. This is because different compounds absorb UV light at different wavelengths, making them visible. Hence it's possible to identify them after the separation process. However, some compounds have little or no UV absorbance, making it difficult to see or even identify them. Such compounds require a different detector, such as the Evaporative Light-scattering Detector (ELSD), to fractionate and identify them.
How ELSD Works
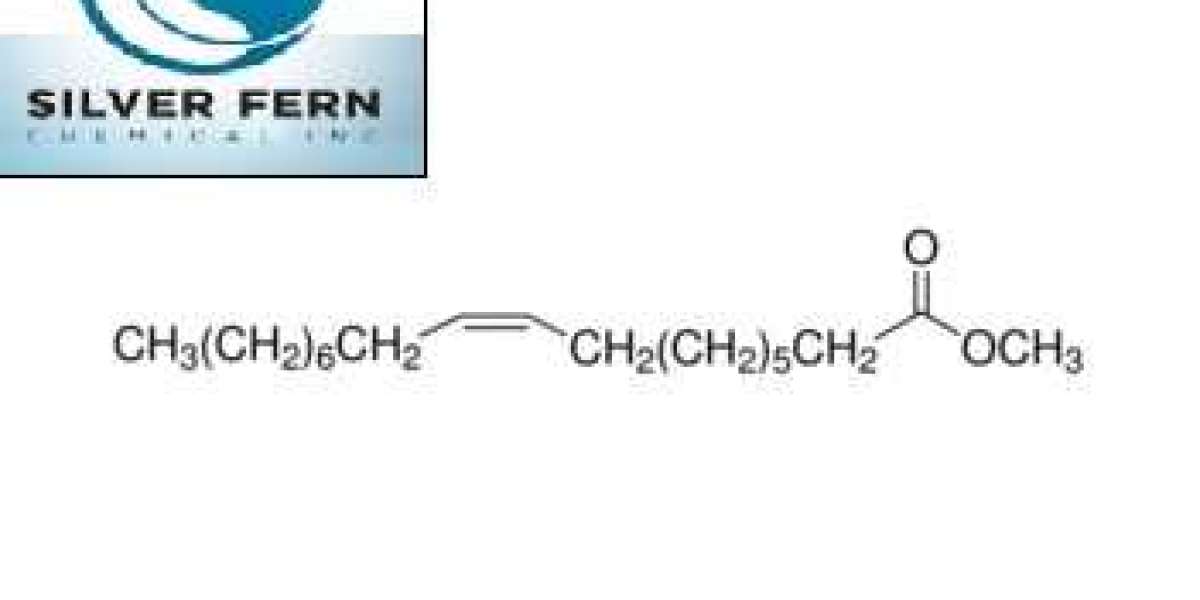
An ELSD is your best bet if your flash chromatography involves complex samples such as carbohydrates, lipids, essential oils and natural products. Others with low UV absorbance, such as methyl oleate, may also require an ELSD.
Unlike other detectors used in chromatography, ELSD doesn't require products to have specific functionality and ionization. It only requires the product not to evaporate under the detector's conditions. These conditions are sensitivity level, inert gas flow, sample inlet flow and setting evaporation temperature. Here's how an ELSD works in flash chromatography.
- Splitting the Effluent
An ELSD requires only a portion of the chromatographic cartridge effluent. Therefore, the first step involves splitting it to get a small amount into the ELSD while the majority proceeds to the fraction collector.
- Nebulizing the Effluent
Once the effluent is in the ELSD, it mixes with the inert gas, usually nitrogen. Then it's heated to a particular temperature until it evaporates.
- Mobile Phase Evaporation
In this phase, the evaporated solvent goes to a heated chamber called a drift tube. The vapor is further heated to ensure the solvent evaporates so the compounds can form air-born particles.
- Particle Detection
Detecting the dried particles is the last phase in the ELS detection process. The particles pass through a light beam scattering it. To identify the particles, you relate the amount of scattering to the mass of the compounds.
ELSD is best used when you need to identify a lot of impurities. This is because it's better at detecting non-chromophoric molecules than the UV detector. In addition, it gives an accurate indication of relative quantities compared to UV. Also, using chromatography instruments with ELSD reduces sample loss by enabling maximal recovery.
ELSD is a crucial instrument in chromatography. It helps in detecting and identifying compounds with low UV absorbance. It's best for purifying complex compounds such as carbohydrates, saturated terpenes, and other molecules with little or no UV-absorbing chromophores. It makes your work easier and faster since it works better than UV. Therefore, an ELSD makes your job easier and quicker despite the sample type.